The security and confidentiality of your personal data is very important to us. More information about our Privacy Policy
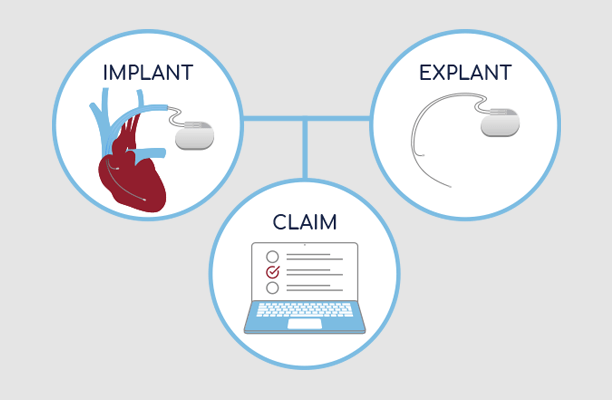
Implant & Explant lifecycle management software.
Minimizing gaps in the implant and explant lifecycle for maximum efficiency, compliance, and resource savings.
We provide comprehensive solutions that bridge the entire implant and explant lifecycle, encompassing tissue, non-biologics, product recalls, explants, and warranty claims. Our software boasts essential integrations to optimize management, increasing efficiency and cost-effectiveness. With streamlined processes and robust built-in compliance safeguards, our solutions not only reduce staff workload but save valuable resources.
Explore InVita's Implant & Explant management product suite.
Maximize efficiency, savings, and ease for smarter implant & explant lifecycle management.
Streamline lifecycle management of medical implants.
InVita’s platform manages tissue and implants throughout the entire lifecycle, automating processes each step of the way. Gaps in the implant supply chain are closed and loss and waste avoided – all contributing to a significant impact on your bottom line.
Automated processes = efficiency & compliance.
InVita’s implant management solution captures data that drives staff efficiency. UDITracker meets and exceeds compliance standards set by The Joint Commission (JCAHO), Food & Drug Administration (FDA), and Det Norske Veritas (DNV).


Key integrations ensure accuracy, patient safety, and cost control.
UDITracker integrates EHR, MMIS, and other vital data that drive optimization and significantly reduce the need for manual data entry. Barcode scanning technology and data from device manufacturers and the FDA global UDI database automate the flow of information and ensure accuracy. Recall matching is automated through the integrations of FDA Recall Database, EHRs, and device manufacturers’ databases.